A promising new method involves allosteric inhibitors – drugs that bind to different parts of the protein, changing its behaviour and preventing harmful interactions…reports Asian Lite News
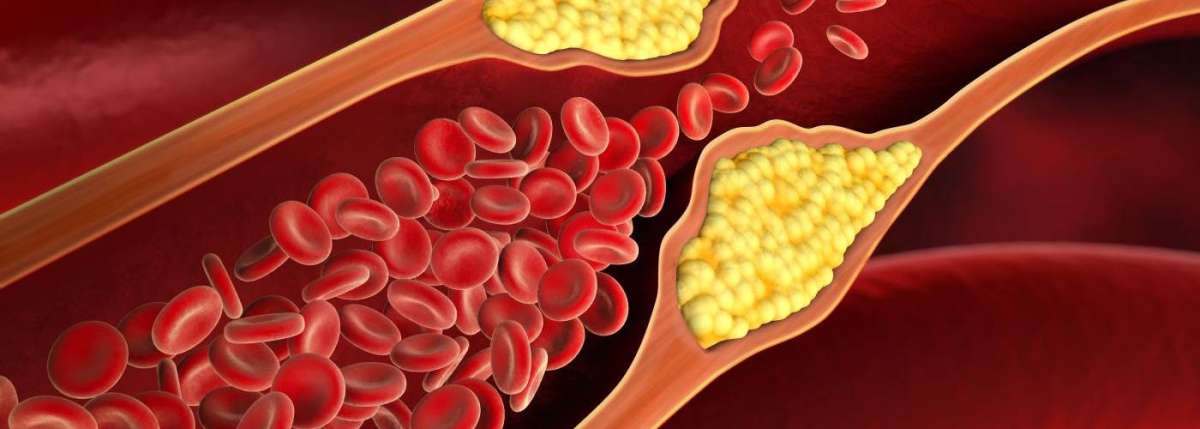
Researchers from S. N. Bose National Centre for Basic Sciences, Kolkata, have explored a new way to manage conditions like elevated low-density lipoprotein (LDL), or cholesterol, levels.
Proteins are vital for our health, performing a wide range of functions. However, incorrect protein interactions can cause diseases. Traditionally, scientists have tried to develop small molecule drugs that act as competitive inhibitors to protein-protein interaction (PPI) sites. However, this has proven difficult due to the large and smooth nature of protein interaction areas.

An alternative approach uses large peptides or antibodies to inhibit PPIs, though these can be costly and difficult to administer.
Pharmaceutical industries thus seek small molecules that are easier to take, typically in pill form.
A promising new method involves allosteric inhibitors – drugs that bind to different parts of the protein, changing its behaviour and preventing harmful interactions.
The challenge is identifying these special target spots on proteins. The researchers propose a new computational protocol to predict and identify alternative binding pockets and hotspots on a protein surface that are allosterically coupled to the functional site using advanced computer simulation approaches.
As a test case, they examined PCSK9, a protein that controls cholesterol levels by interacting with low-density lipoprotein receptor (LDLR). Increased PCSK9-LDLR interaction can elevate LDL levels, contributing to heart disease. Current treatments targeting PCSK9 are expensive and not suitable for everyone. Finding an orally administered small-molecule drug that blocks the PCSK9-LDLR interaction could be transformative.

Dr Suman Chakrabarty’s team has made significant progress in identifying targetable parts of the PCSK9 protein. They used thermodynamics to argue that the bidirectional nature of allostery can identify allosteric pockets. By comparing the conformational ensembles of bound and unbound protein states, they propose targeting unique conformations in the unbound state for drug discovery. This collaborative approach between academia and industry aims not only to lower cholesterol but also to create a new paradigm in drug design, targeting proteins more effectively to prevent diseases.
ALSO READ-Healthy options to replace high cholesterol snacks